Research
Research
Vedoucí oddělení:
M.Sc. Tereza Toralová, Ph.D.
|
|
|
Regulation of mammalian oocyte maturation and preimplantation embryo development
The Laboratory of Developmental Biology focuses on gene and protein expression during oocyte meiotic maturation and early embryonic development of mammals. We use pig and bovine oocytes and embryos cultivated in vitro as experimental models.
Our research interests are primarily concentrated on three following areas:
1) Identification of genes and signaling pathways regulating oocyte maturation and developmental competence
2) Mechanisms of embryonic genome activation during the preimplantation development
3) Molecular studies on the role of oocyte nucleolus in regulation of embryo development
Genes and signaling pathways regulating oocyte maturation and developmental competence
During the growth phase, the oocyte is arrested in the first meiotic prophase, but it gains full meiotic competence in several steps. However, these meiotically competent oocytes still remain in the dictyate stage of the prophase if they are retained within follicles. They only resume meiosis following the preovulatory surge of luteinizing hormone (LH) or after the release into a suitable culture medium. The resumption of meiosis is regulated inside oocytes by a post-translation mechanism and does not depend on the activation of genes in the oocyte itself. Nevertheless, the LH-induced meiotic resumption is accompanied by a dramatic change in gene expression profiles in mural granulosa and cumulus cells. The changes in the cumulus/granulosa cell transcriptome are under the control of a broad signaling network activated in the follicular cells by the LH surge.
Oocyte maturation
We have been studying molecular mechanisms regulating oocyte meiotic arrest and meiotic resumption with a special attention to somatic follicular cell and oocyte intercellular communication. By the use of real-time polymerase chain reaction, microarray analysis and immunoblotting, we have identified on a model of pig cumulus-oocyte complexes genes and proteins that are involved in regulation of oocyte meiotic maturation. A special attention was paid to characterization of signaling molecules and signaling cascades that are activated in preovulatory follicles by gonadotropin hormones and modified by locally produced EGF-like peptides (Procházka et al., 2012; Blaha et al., 2015).
Oocyte developmental competence
The developmental competence of oocytes is acquired progressively during folliculogenesis and is linked to follicular size. It has been documented that oocytes originating from larger follicles exhibit a greater ability to develop to the blastocyst stage. The differences in cytoplasmic factors such as mRNA transcripts could explain the differences in oocyte developmental potential. We used bovine oligonucleotide microarrays to characterize differences between the gene expression profiles of germinal vesicle stage oocytes with greater developmental competence from medium follicles (MF) and those with less developmental competence from small follicles (SF). We found differences in the level of 60 transcripts (≥1.4 fold), corresponding to 49 upregulated and 11 downregulated transcripts in MF oocytes compared to SF oocytes. The majority of the genes were associated with the regulation of transcription, translation, the cell cycle, and mitochondrial activity. It can be concluded that the developmental competence of bovine oocytes and embryos may be a quantitative trait dependent on small changes in the transcription profiles of many genes (Němcová et al., 2016). Further activities of the laboratory are aimed to improve culture systems for mammalian oocytes and recognition of high-quality oocytes by molecular methods.
Embryonic genome activation during the preimplantation development
Preimplantation development of mammals is characterized by three major developmental transitions, which occur after fertilization of oocyte – transition from oogenetic to embryonic genomic control (or zygotic gene activation – ZGA), compaction (which results in the formation of polarized epithelium) and differentiation of the morula into the blastocyst The ZGA exhibits a pattern that includes the degradation of maternal mRNA in co-ordination with the onset of transcription and subsequent translation of mRNA from the embryonic genome. This switch occurs very early at mice; zygotic genome is fully activated at the stage of 2 cells. This major activation is even preceded by transcription starting already in male pronucleus at one cell stage. Similar situation was found even in bovine embryo where genome is fully activated by the late 8–cell stage and there is some evidence that minor activation is already turned on at the 2–cell stage. In contradiction to a mouse, where oocyte meiotic maturation and reprogramming of gene expression has been well described, there is still a variety of unresolved questions in porcine and bovine species, which represent better model for human oogenesis and embryogenesis than rodents.
Role of nucleophosmin
Nucleophosmin was chosen for our study as a gene potentially crucially important for bovine preimplantation development. We have found that embryonic transcription of nucleophosmin starts during the major wave of embryonic genome activation, which indicates its necessity for bovine preimplantation development. The mRNA expression level of nucleophosmin was found to decrease from MII stage to early 8-cell stage and started to increase at late 8-cell stage. This increase was α-amanitin sensitive and thus represents the start of transcription from embryonic genome. Consequently, we monitored the influence of nucleophosmin mRNA silencing on the early embryogenesis. In summary our data have shown that a small amount of nucleophosmin protein is preserved throughout whole preimplantation development and enables the development of embryos until the blastocyst stage (Toralová et al., 2012).
SCF complex
Currently, our most important research concerns ubiquitination during bovine preimplantation development. We concentrate on Skp1-Cullin1-Fbox (SCF) complex, the expression of its invariant components (Cullin 1, Skp1, Rbx1) and its contribution to degradation of maternal proteins. Our up to now results have shown that embryonic expression of all these genes starts in initial stages of development. Especially Cullin 1 is activated very early, already at 4-cell stage. Genes participating in ubiquitination are usually activated at 8-cell stage, and the early activation of Cullin 1 suggests its necessity for embryonic genome activation. Protein localization analysis showed interesting results especially at the blastocyst stage. There was clear concentration of protein expression and SCF complex activation to trophectoderm. These results allowed us to initiate the experiments dealing with the application of SCF complex during preimplantation development and especially in maternal protein degradation (Benešová et al., 2016).
Molecular studies of nucleolar inheritance
The nucleolus is the cell organelle responsible for ribosome synthesis and, hence, for protein synthesis. In the mammalian oocyte, the nucleolus compacts into a dense sphere (nucleolar sphere) without ribosome-synthesizing capacity well in advance of ovulation.
Elegant studies in pig and mouse, where the oocyte’s nucleolar spheres were removed (enucleolation) or even exchanged by micromanipulation, demonstrated that the nuclear sphere is completely essential both for completion of the oocyte’s meiosis and for further embryonic development. For example, oocytes that had their nucleolar sphere removed did not sustain embryonic development. However, if another nucleolar sphere was transplanted to replace the lost one, embryonic developmental capacity was restored. Interestingly, if a nucleolus from a somatic cell or even an embryonic stem cell was used for the transplantation, the oocyte again did not sustain embryonic development. Hence, the components conveyed from the oocyte’s nucleolar sphere to the embryo are not merrily nucleolar material, but includes other biomolecules that are crucial for the embryo to develop. Hence, based on these studies the oocyte’s nucleolar sphere is indeed required for conception and further life. It is, however, unknown what the nucleolar sphere contains and in what way it contributes to embryonic development. In our project, the pig oocytes and embryos will be used to study the content of the nucleolar sphere as well as the developmental competence conveyed by this structure. The content of the sphere will be analyzed by transcriptomics and proteomics, and the developmental competence of the sphere tested by advanced micromanipulation where nucleolar spheres will be transplanted from one egg to another.
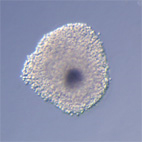 |
Oocyt-kumulární komplexy prasete po kultivaci in vitro v médium s dbcAMP. |
 |
Cover: Confocal laser scanning microscopy of tubulin polymerization of bovine embryo after immunofluorescence analysis. Alpha-tubulin (green), nucleophosmin (red), nuclei (blue). Toralová et al. 2012, Bovine preimplantation embryos with silenced nucleophosmin mRNA are able to develop until the blastocyst stage. Reproduction 144, 349-359. |
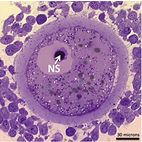 |
Histological section of a pig oocyte-cumulus complex with highlighted nucleolar sphere. |
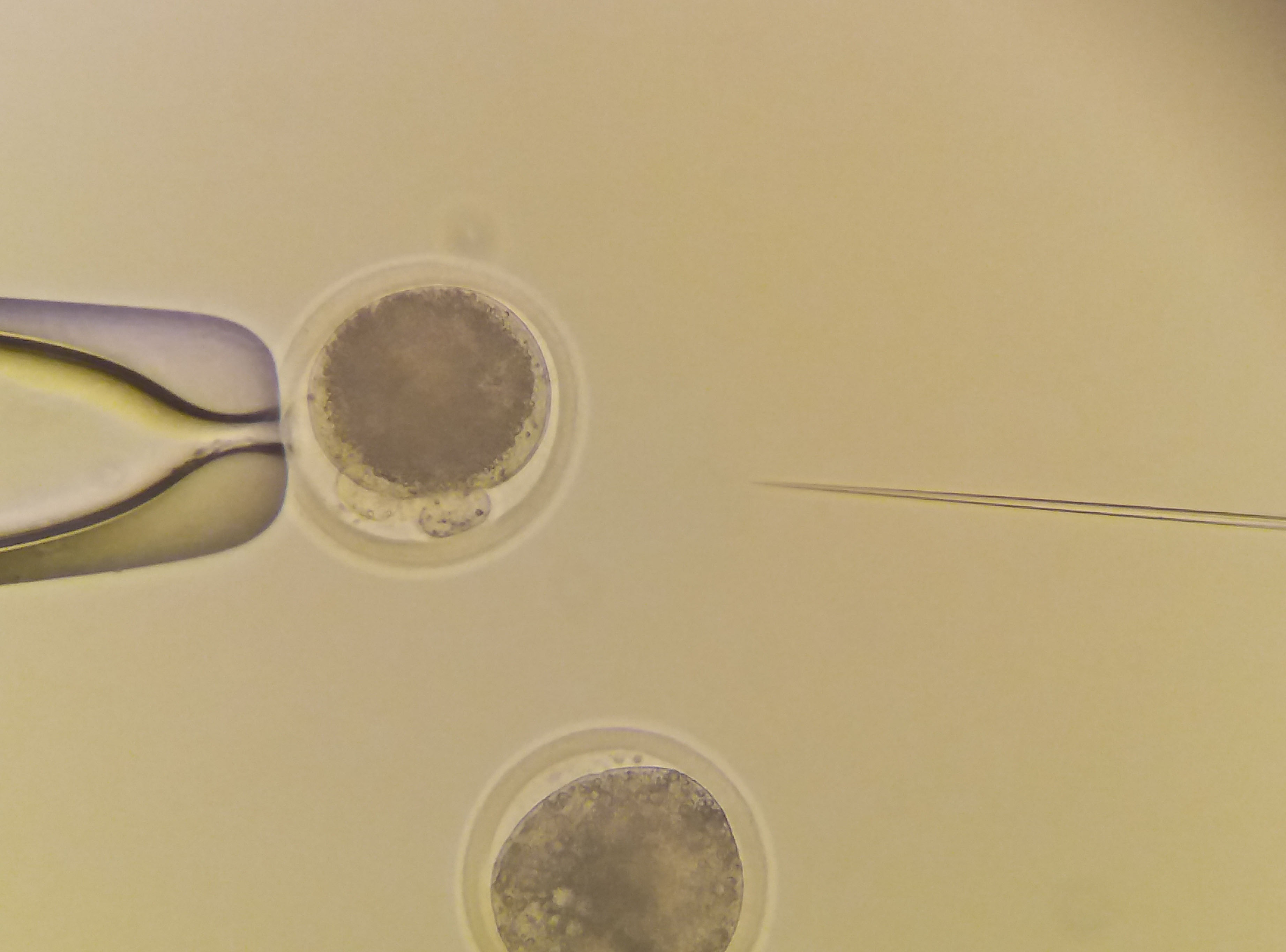 |
Microinjection to bovine zygotes. |
| Detection of F-actin in bovine oocyte surrounded by cumulus cells. F-actin - green, DNA - blue. | |
| Detection of protein localization (PIASy) in bovine GV oocyte with nuclear localization in detail. Red - protein, blue - DNA. |
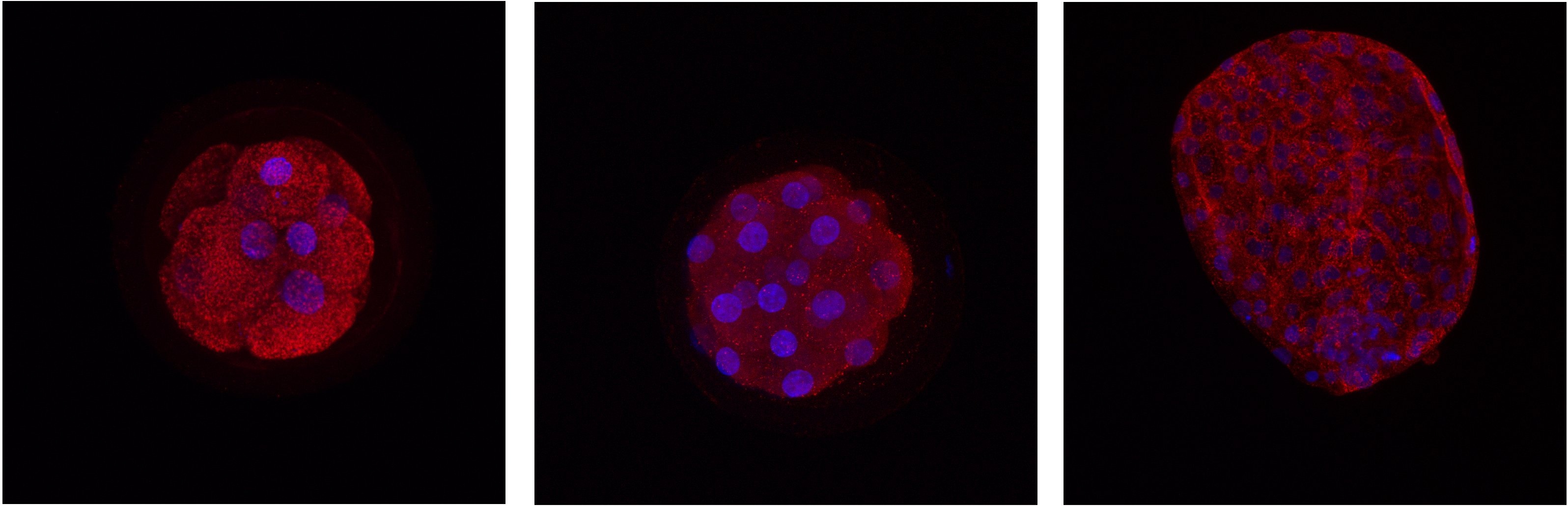
Detection of protein localization in different stages of bovine embryo. Red - protein, blue - DNA.